Chlorine + Sodium Bromide . Cl 2 (aq) + 2nabr (aq). if chlorine is added to a solution of sodium bromide, a reaction occurs: Chlorine + sodium bromide → bromine + sodium. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Chlorine + sodium bromide → sodium chloride + bromine. in this equation, the cl and br have swapped places: what is the net ionic equation for the displacement reaction between aqueous. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions.
from www.chegg.com
nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. in this equation, the cl and br have swapped places: what is the net ionic equation for the displacement reaction between aqueous. Chlorine + sodium bromide → bromine + sodium. use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. if chlorine is added to a solution of sodium bromide, a reaction occurs: Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq).
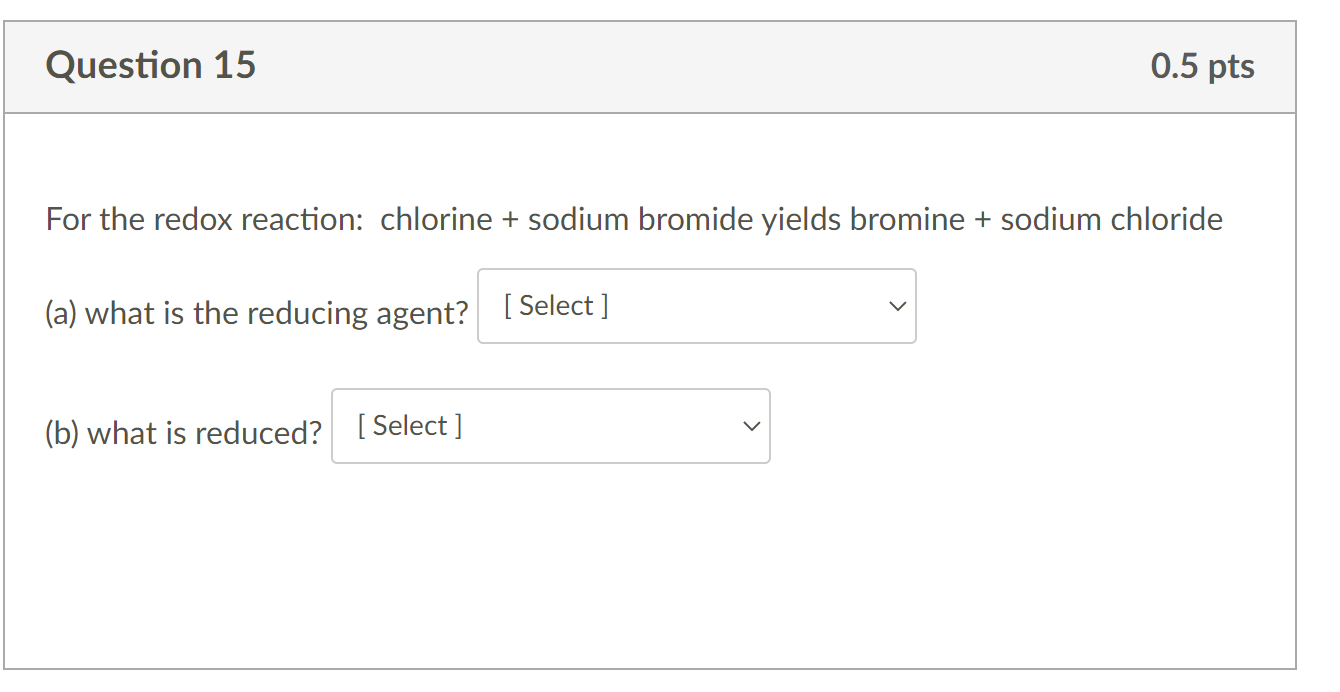
Solved For the redox reaction chlorine + sodium bromide
Chlorine + Sodium Bromide nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. Chlorine + sodium bromide → bromine + sodium. what is the net ionic equation for the displacement reaction between aqueous. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. if chlorine is added to a solution of sodium bromide, a reaction occurs: nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. in this equation, the cl and br have swapped places: Cl 2 (aq) + 2nabr (aq). Chlorine + sodium bromide → sodium chloride + bromine.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector (Royalty Free) 1977753731 Chlorine + Sodium Bromide what is the net ionic equation for the displacement reaction between aqueous. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. use the teat pipette. Chlorine + Sodium Bromide.
From fphoto.photoshelter.com
science chemistry halide displacement reaction chlorine postassium bromide Fundamental Chlorine + Sodium Bromide nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Cl. Chlorine + Sodium Bromide.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chloride+Bromine YouTube Chlorine + Sodium Bromide use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. if chlorine is added to a solution of sodium bromide, a reaction occurs: what is the net ionic equation for the displacement reaction between aqueous. nabr + cl = br + nacl is a single. Chlorine + Sodium Bromide.
From www.numerade.com
SOLVED 38. Write balanced chemical equation for the following reaction Chlorine gas reacts Chlorine + Sodium Bromide Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq). if chlorine is added to a solution of sodium bromide, a reaction occurs: use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. nabr + cl2 = nacl + br2 is. Chlorine + Sodium Bromide.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Chlorine + Sodium Bromide Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq). in this equation, the cl and br have swapped places: what is the net ionic equation for the displacement reaction between aqueous. Chlorine + sodium bromide → bromine + sodium. nabr + cl = br + nacl is a single displacement (substitution). Chlorine + Sodium Bromide.
From londonstatus.co.uk
Why Does Chlorine Displace Bromine from Sodium Bromide? London Status Chlorine + Sodium Bromide use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq). what is the net ionic equation for the displacement reaction between aqueous. nabr + cl = br + nacl is a. Chlorine + Sodium Bromide.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Chlorine + Sodium Bromide in this equation, the cl and br have swapped places: nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. what is the net ionic equation for the displacement reaction between aqueous. Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq).. Chlorine + Sodium Bromide.
From slideplayer.com
Chemical Equations Chapter ppt download Chlorine + Sodium Bromide use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. what is the net ionic equation for the displacement reaction between aqueous. in this equation, the cl and br have swapped places: Chlorine + sodium bromide → bromine + sodium. if chlorine is added to. Chlorine + Sodium Bromide.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are formed by the reaction of Chlorine + Sodium Bromide what is the net ionic equation for the displacement reaction between aqueous. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. Chlorine + sodium bromide → sodium chloride + bromine. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole. Chlorine + Sodium Bromide.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Chlorine + Sodium Bromide Chlorine + sodium bromide → bromine + sodium. Cl 2 (aq) + 2nabr (aq). nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. if chlorine is. Chlorine + Sodium Bromide.
From www.shutterstock.com
Sodium Salts (Set 2) Sodium Fluoride, Chloride, Bromide, Iodide, Hydride, Hydroxide, Cyanide Chlorine + Sodium Bromide in this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium chloride + bromine. use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of. Chlorine + Sodium Bromide.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Chlorine + Sodium Bromide use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. Chlorine + sodium bromide → sodium chloride + bromine. if chlorine is added to a solution of sodium bromide, a reaction occurs: what is the net ionic equation for the displacement reaction between aqueous. Cl 2. Chlorine + Sodium Bromide.
From www.youtube.com
Chlorine Water + Sodium Bromide YouTube Chlorine + Sodium Bromide if chlorine is added to a solution of sodium bromide, a reaction occurs: Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. Cl 2 (aq) + 2nabr (aq). . Chlorine + Sodium Bromide.
From www.numerade.com
When chlorine gas is bubbled into a solution of sodium bromide, the sodium bromide reacts to Chlorine + Sodium Bromide nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two moles of aqueous sodium bromide. Chlorine + sodium bromide → sodium chloride + bromine. what is the net ionic equation for the displacement reaction between aqueous. if chlorine is added to a solution of sodium bromide, a reaction occurs: Chlorine + sodium. Chlorine + Sodium Bromide.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a solution of silver nitrate Chlorine + Sodium Bromide if chlorine is added to a solution of sodium bromide, a reaction occurs: use the teat pipette to slowly add the same volume (1 cm) of chlorine water to each of the halide solutions. Chlorine + sodium bromide → bromine + sodium. nabr + cl2 = nacl + br2 is a single displacement (substitution) reaction where two. Chlorine + Sodium Bromide.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas) YouTube Chlorine + Sodium Bromide what is the net ionic equation for the displacement reaction between aqueous. Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. if chlorine is added to a solution of sodium bromide, a reaction occurs: in this equation, the cl and br have swapped places: Cl 2 (aq) + 2nabr. Chlorine + Sodium Bromide.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to produce sodium bromide Chlorine + Sodium Bromide what is the net ionic equation for the displacement reaction between aqueous. Chlorine + sodium bromide → sodium chloride + bromine. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Cl 2 (aq) + 2nabr (aq). Chlorine + sodium bromide → bromine + sodium. use the. Chlorine + Sodium Bromide.
From slidetodoc.com
Describing a Chemical Reaction Indications of a Chemical Chlorine + Sodium Bromide Cl 2 (aq) + 2nabr (aq). in this equation, the cl and br have swapped places: if chlorine is added to a solution of sodium bromide, a reaction occurs: nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Chlorine + sodium bromide → sodium chloride +. Chlorine + Sodium Bromide.